The challenges of a cell & gene therapy supply chain - part 4


Designing high-performance, risk-controlled, and scalable cell & gene supply chains with a digital twin
[PART 4] - Forecasting CGT demand and adapting the supply chain
In the preceding articles of this series [part 1 - part 2- part 3] we have shown how the optimal manufacturing capacity is determined by the dynamics of demand and supply.
Accurately evaluating demand is important not only for installing the right manufacturing capacity but also for scaling the distribution. Many cell & gene therapies (CGT) can hardly be distributed via the classical pharmaceuticals distribution network of wholesalers and pharmacies, where local safety stocks are anticipatively built to cope with the demand variability, because of their:
- high price tag
- low-temperature conditioning
- low product stability
- limited availability
As CGT are shipped just-in-time, properly sizing the distribution capacity requires a sound evaluation of demand. Still, predicting the demand for cell & gene therapies may be challenging, both at the clinical and commercial stages.
Clinical demand
There is a large consensus that planning for the distribution of investigational medical products represents a major challenge. This is because, beyond the variability of supply chain operations, clinical research is subject to extra sources of unknown, for example:
- Clinical protocols are random by design
- Product efficiency or optimal dose is often unknown
- Product shelf-life is limited
- IMP and comparators are expensive and difficult to source
- The patient population is limited and their location is poorly predictable
- As the manufacturing process is not fully optimized yet, quality and yield are more uncertain
Most of these features are exacerbated by the nature of cell & gene therapies as discussed in the first part of this series (e.g. short shelf-life, high cost, small patient population, complex manufacturing and distribution).
Over the last decade, N-SIDE has helped emerging biotechs and established pharmaceutical companies like Sanofi optimize their strategy and operations by means of the N-SIDE Supply app, enabling them to:
- Minimize waste
- Control costs
- Reduce time-to-market
- Progress towards sustainability
- Reduce risk
- Manage production plans
These results were only made possible by using stochastic digital twins. Indeed, a deterministic forecasting application would barely cascade the needs in time and quantity, which forces planners to think in terms of worst-case demand and subjective buffers. There are 2 major issues with this approach:
- There is no such thing as a single worst-case scenario. For example, considering 1 patient per site per month, it is tempting to schedule the patient's arrival on the 1st day of the month to ensure the IMP will always be delivered early enough. However, in order to verify the product expiry, supply chain managers need to consider the latest possible treatment date, on the last day of the month. Consequently, neither of these approaches adequately covers the supply chain risks.
- Taking into account all possible adverse events without any consideration for their probability of occurrence results in oversized safety stocks, which eventually generate dispensable waste and costs, not to mention the extra subjective buffers that are usually added on top of calculated needs.
Conversely, a stochastic model (Exhibit 1) will:
- Consider that 1 patient on average means 0, 1, 2, 3, or any number, albeit with very different probabilities, and that these patients can be treated anytime in the month.
- Repeat the sampling process thousands of times to ensure the entire spectrum of possible demand is taken into account to generate an optimal and robust execution plan
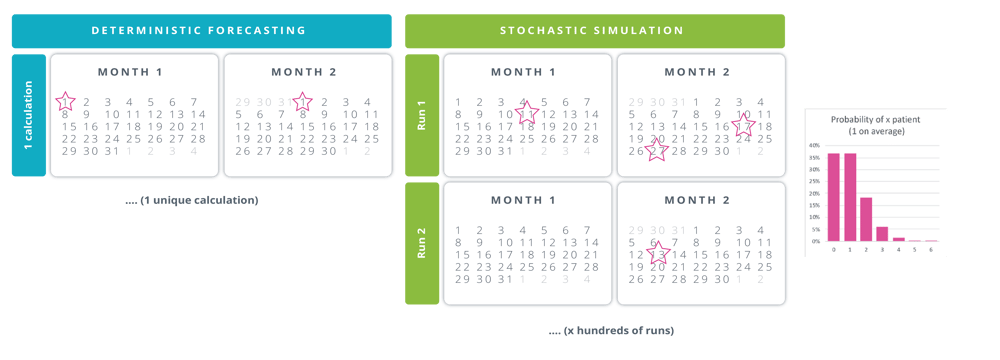
[Exhibit 1] Comparison of deterministic and stochastic approaches to modeling demand
By embedding a stochastic modeling approach, the N-Side Supply app typically results in:
- 20-50% costs reduction
- 20-60% waste reduction
- 100% service level
Commercial demand uptake
Even in a commercial context, establishing demand remains a challenge, especially in the case of one-off curative treatments.
Indeed, the demand for a regular drug increases until it reaches a plateau at maturity. In the case of one-off therapies, however, the initial pool of pre-existing patients is depleted over time until eventually, when all pre-existing patients are cured, the demand is only determined by the flow of new patients. Consequently, there is a peak in the number of patients treated, whose intensity and position in time depends on the pace at which the patient pool is addressed (Exhibit 2).
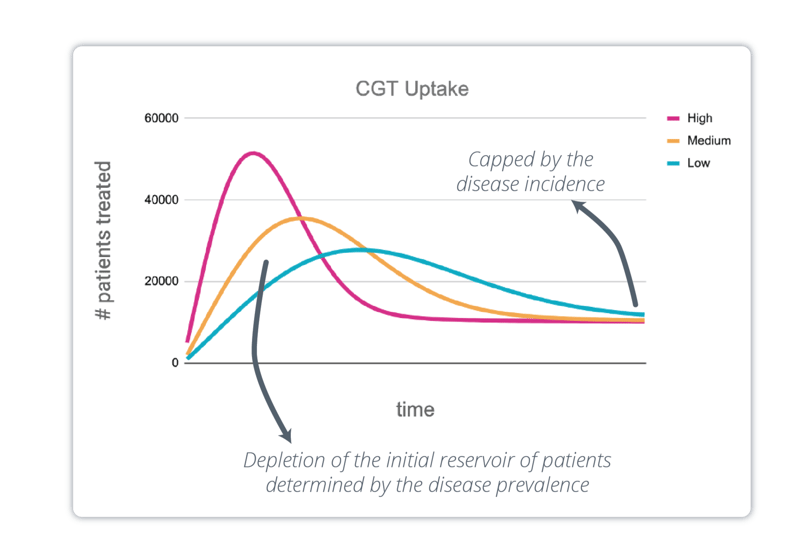
[Exhibit 2] CGT uptake with high/medium/low % of market captured
Ideally, demand and capacity should continually match. This can be approached by:
- Dynamically adapting the capacity, for example by:
- Subcontracting operations
- Scaling up the manufacturing process in useful time
- Reallocating resources to another product
- Modulating demand, for example by:
- Increasing the geographic size of the market
- Developing new product indications
Whichever strategies are envisioned, any supply chain design should therefore:
- Consider the dynamics and variability of demand
- Run sensitivity around the assumptions
- Perform what-if analyses for the various scenarios
Conclusion
Realizing a CGT full potential requires an early determination of the best possible operating supply chain. Because CGTs are particularly prone to variation, digital twin models leveraging stochastic modeling provide supply chain managers with valuable insights into operational performance and help them make informed decisions.



About the Author
Philippe has been helping pharma and biotech companies create and operate their supply chains for more than 20 years. Before joining N-SIDE as Strategic Project Leader, he directed the supply chain of a cell therapy company for 8 years and gained a firsthand experience of the specific challenges of this technology. He obtained a PhD in Bioengineeing, by creating a computational model of protein/membrane interactions. He also holds a certificate in Management for Biomedical Industry Executive.
Philippe Ducarme




Other content for you

article
Explained: Forecasting and Optimization in Clinical Trials
Growing challenges related to clinical trial management have renewed interest in forecasting and optimization solutions.
Many pharmaceutical companies and contract research organizations (CROs) are examining their existing forecasting and optimization processes and searching for more effective solutions.
Read more
blog post
The 3 biggest challenges in the clinical trial supply chai
Drug supply chain management is a balancing act. You need to ensure enough drug is available to meet patient demand and that supply is allocated where it’s most needed. Meanwhile, you have to minimize costs and timelines while also preparing for the unexpected.
Today, the three biggest challenges in the clinical trial supply chain are uncertainty, siloed decision-making, and supply bottlenecks.
read more
white paper
End-to-end clinical supply chain optimization helps bring drugs to market faster
With a growing global population, speeding up drug development timelines is of paramount importance for all pharmaceutical and biotech companies. Thanks to innovative software solutions it’s now possible to make better decisions at each stage of the clinical supply chain.
download now
