What is a clinical trial supply risk management plan?


When we talk about risk management in clinical trial supply, we’re usually referring to two types of risk:
First, there’s the risk that patients will miss dispensing because the drug they need isn’t available on-site on the day they need it. Missed dispensing can negatively impact patient health by delaying a specific patient’s treatment, throwing off the results of the trial, and causing patients to drop out. Typically, the impacts of missed dispensing events are short-term and affect a specific site and a small number of patients.
Second, there’s the risk of global drug shortage, a.k.a. lacking drugs not just at the site, but also not having any in depots to pull from. Drug shortages have long-term impacts and may lead to a large number of patients missing their treatments, threatening the success of the trial.
Risk of missed dispensing is generally easier to control than the more consequential risk of a global drug shortage. Ensuring that both types of risk are mitigated is critically important.
Definition of a risk management plan in clinical supply
A clinical trial supply risk management plan is an approach to preventing or dealing with shortages in drug supply in clinical trials.
There are two types of clinical trial supply risk management plans: proactive and reactive.
Proactive risk management plans
Proactive risk management plans are initiated months before the trial starts. The goal is to ensure decisions are made that lead to an optimal supply chain and keep risk as low as possible.
Creating a proactive clinical trial supply risk management plan requires using the right systems and data to simulate and forecast the trial, then using the insights gained to optimize the supply strategy.
For example, supply managers may be able to foresee moments in the trial when demand could be higher than expected, and plan to accommodate that excess demand by increasing production in those moments.
Supply managers must strike a careful balance between risk management and waste. While there should always be enough drug to cover uncertainty in demand, calling for too much can lead to inflated costs and timelines. The overage is therefore one of the important outputs of a proactive risk management plan.
A proactive risk management plan should also involve looking at the trial design and identifying points where drug consumption can potentially be decreased with adjustments to strategic decisions such as protocol, manufacturing, kit design, vendor selection, and country selection.
The plan always includes the site resupply strategy, as well as configuring the IRT system in a way that makes it robust enough to tackle uncertainty in demand, reducing the risk of missed dispensing.
Finally, this plan should include an operating procedure for monitoring the trial in order to identify anomalies as soon as they happen and take immediate action to avoid missed dispensing and shortages.
Reactive trial supply risk management plans
Reactive risk management plans detail how to respond to missed dispensing and drug shortage events once they’re about to happen or are currently happening.
While a good proactive plan will mitigate risks, there is always a possibility of a shortage, so it’s necessary for supply managers to be prepared. Disruptions to the supply chain can be caused by a number of factors, including:
- Not having a proactive risk mitigation plan
- Delays in batch releases, lost batches, and lower yields in manufacturing
- Extremely fast recruitment or much higher demand than expected
- Shortage of comparators in the commercial market
Without access to a reactive risk management plan, it’s easy to panic and make rash decisions in the event of missed dispensing or shortages. The typical responses include changing IRT configuration, stopping recruitment, or closing sites — but actioning the latter two responses without careful consideration can delay the trial timeline more than necessary.
Reactive risk management plans can help guide decision-making when problems occur, but this should be the last line of defense.
Reactive risk management is not a substitute for proactive risk management, and relying on a reactive risk management plan will typically lead to uncertainty, stress, and long hours for the supply team.
Learn more about reactive risk management plans
Good practices to create a clinical trial supply risk management plan
N-SIDE has been helping clients create both proactive and reactive clinical trial supply risk management plans for two decades. Using the N-SIDE Supply App and risk-based optimization techniques, we can help shape your plan around real risk data rather than gut feelings.
Managing risk before a trial begins
If you’re still planning your clinical trial, N-SIDE can help you develop a proactive risk management plan that dramatically reduces the risk of missed dispensing and drug shortages, as well as minimizing how much you’ll need to rely on reactive risk management later.
Our proactive clinical supply risk management method includes the following:
- Starting with robust, accurate demand simulations in the N-SIDE Supply App, which factor in uncertainty to produce risk-based optimization recommendations
- Assessing how your supply chain can be set up to cover the risk of worst-case scenarios and plan out how to course correct if needed
- Assessing the impact of early trial design decisions (including protocol design, CMO selection, IRT selection, network selection, and country selection) and recommending adjustments to reduce risk
- Configuring the IRT settings to cover uncertainty in demand
The N-SIDE Supply App also helps supply managers monitor the trial and compare real conditions to forecasted conditions, making it easier to identify risks and act quickly before they escalate.
With a visual dashboard, you can see abnormalities in the demand, recruitment, and inventory data. You can even receive automated alerts if the real-time data exceeds the maximum forecast. This type of monitoring can shave months off of reaction times to unexpected events.
Managing risk for trials in progress
If your trial is already in progress, it’s not too late to take advantage of some of the benefits of N-SIDE’s software and professional services.
When an unexpected event occurs that could lead to missed dispensing or a drug shortage, we can use the N-SIDE Supply App to evaluate your response options and choose the approach that will efficiently mitigate the risk while having the smallest impact on the trial timeline.
To do this, we first analyze the source of the risk by determining the likelihood of shortages impacting the trial, quantify the risk, and determine where and when it might happen, what drug types are impacted, and how many patients could be affected.
There are actually hundreds of potential responses to risk in clinical trial supply. We list and prioritize them based on the following variables:
- Timeline to implementation
- Number of stakeholders involved
- Regulatory considerations
- Chances of success
- Cost of implementation
- Feasibility/desirability of a solution
Then we assess each response by its impact on mitigating the shortage. One by one, we will simulate solutions until the risk is completely under control, and make our plan with a selected number of solutions. This helps avoid going too far in mitigation and creating unnecessary costs, delays, time investments, and issues.
By testing responses with the help of N-SIDE, you can typically avoid slowing or stopping recruitment as a result of a supply bottleneck.
Get in touch with our experts to find out how we can help with your clinical trial supply risk management plan.



About the Author
Amaury is N-SIDE's Strategy Advisor for clinical supply chain solutions. Within his 9 years in the industry, Amaury’s objective has been to revolutionise planning and systems to make clinical supply chain more efficient, more ethical, less wasteful and more patient-centric.
Amaury Jeandrain




You might also like ...

Solution
Supply App
The N-SIDE Supply App is the only solution that adds a risk-based optimization approach to clinical supply forecasting.
Make data-driven decisions about overage, packaging, sourcing, IRT setup, depot shipments, and more, all while measuring the precise impact of these decisions on patient service level and budgets.

article
IRT optimization in clinical trials: achieving maximum efficiency
Clinical trial supply managers work hard to ensure trial protocols, supply plans, and resupply strategies are designed for maximum efficiency.
But there’s another way to improve efficiency that’s often overlooked: optimizing the IRT strategy.
read more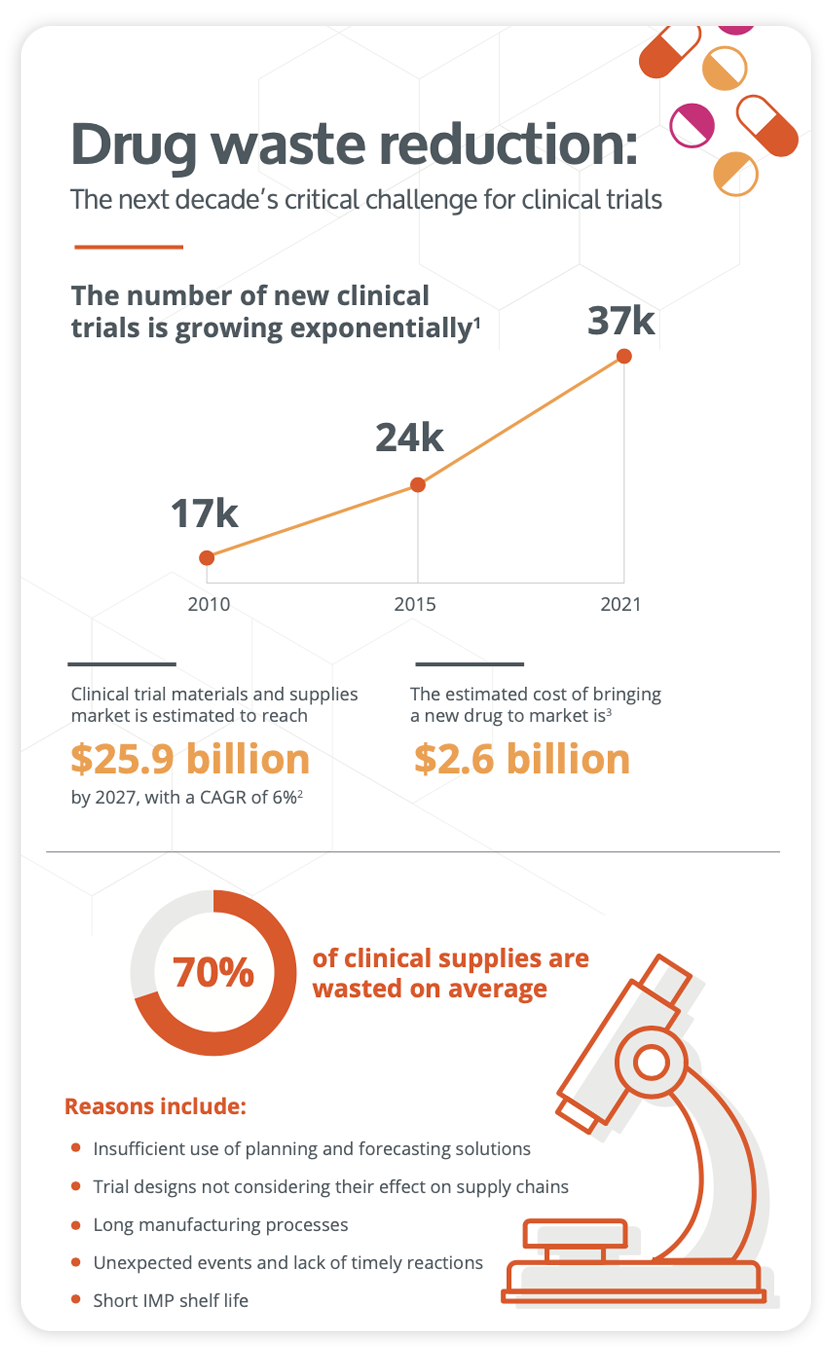
Infographic
Curb drug waste and improve your clinical trial strategy
Download this infographic to learn about the different challenges you might face and how you can streamline your clinical trial strategy thanks to the help of our technology. Waste mitigation and all its associated rewards can indeed be achieved with N-SIDE's clinical supply solutions for the benefit of all stakeholders.
download now



