Clinical supply forecasting & risk-based optimization: What’s the difference?


 Sylvia Haller
Sylvia Haller
As clinical trials have become more complex and expensive, many solutions have come on the market to address the challenges of managing the clinical trial supply chain.
These solutions share a common goal: help clinical trial supply teams get the right amount of drugs to the right locations at the right times, so that no patient in the trial ever misses a dose.
But different solutions go about solving this problem in different ways.
The two major categories of solution in this space are clinical supply forecasting and risk-based clinical supply optimization.
In this article, we’ll explain the difference between these categories and explore which solutions are best in common situations.
What is clinical supply forecasting?
Clinical supply forecasting tools aim to predict patient demand in a clinical trial. The outputs are average demand forecasts that clinical trial supply teams use to create their supply plans.
Clinical supply forecasting tools are typically software products that are either built into or integrated with other trial management technologies.
While some solutions include extra features like scheduling, reporting, and notifications, at their core these tools are complex calculators. They process inputs like anticipated recruitment, titrations, drug expiration, and desired overage through an algorithm in order to produce a forecast.
Clinical supply forecasting tools are very good at working with known variables and producing accurate forecasts. However, they’re not intelligent enough to accurately factor in uncertainty.
Because they don’t consider uncertainty, forecasting tools have two main drawbacks.
- These tools aren’t able to calculate the risk associated with each forecast (i.e., the likelihood that demand will be much higher or lower than forecasted). Forecasts may be accurate on average, but they’re neither robust nor secure in terms of risk.
- Without calculating risk, these tools can’t calculate required overage. Instead, overage must be included as an input in the calculation (usually using a rule of thumb or comparable past trial data). In most cases, this leads to a high level of drug waste.
The advantage of clinical supply forecasting tools is that they can provide rough estimates of demand early on in the planning stages of a trial.
When should you use clinical supply forecasting?
Clinical supply forecasting tools are suitable for use:
- During high-level planning of trials well before study start
- For long-term DS/DP planning using studies with protocols that aren’t fully known or stabilized
- For simple Phase 1 trials where variability is low
What is risk-based clinical supply optimization?
Unlike clinical supply forecasting tools which only predict average demand, risk-based optimization tools use simulations to anticipate uncertainty and risk to the trial supply.
Risk-based clinical trial supply optimization tools leverage different algorithms to model uncertain parameters and simulate possible outcomes of a trial using Monte-Carlo simulations.
After running thousands of simulations, these tools can predict not only average demand, but minimum and maximum demand (or confidence interval) as well.
The uncertain parameters modeled in these simulations typically include:
- Recruitment speed
- Patient dropout
- Titration probabilities
- Visit intervals
- Patient weight
Whereas forecasting tools require overage as an input, risk-based optimization tools produce overage as an output.
This is accomplished by measuring the actual variability of demand, its amplitude, and when and where it occurs. With this information in hand, clinical trial supply teams can proceed with the lowest amount of drug overage necessary to guarantee zero risk of a stockout.
In addition, risk-based optimization tools are used to optimize IRT setup (e.g. safety stocks, trigger levels, resupply frequency), depot resupply strategy, and comparator sourcing strategy.
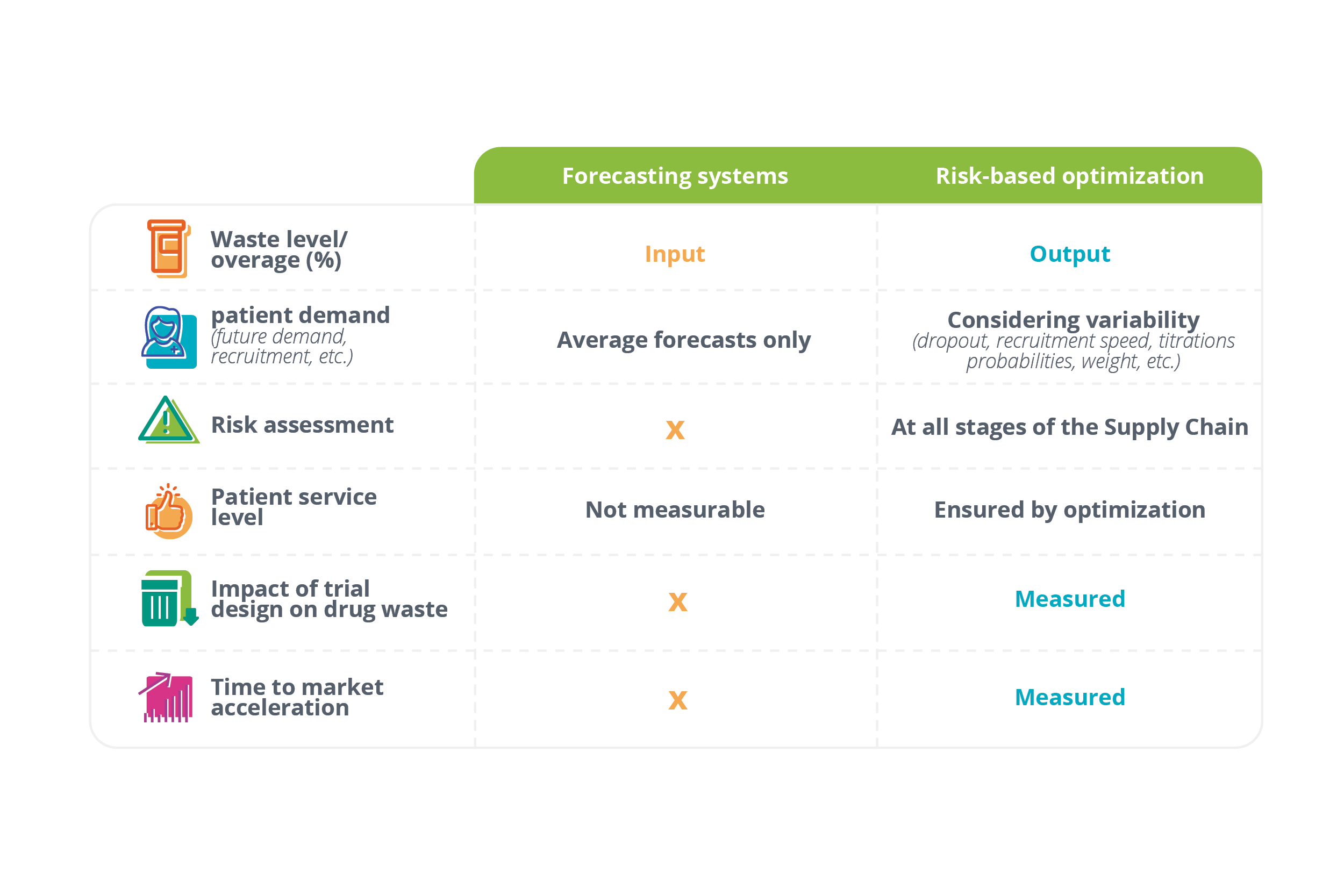
Some of the differences between forecasting and risk-based optimization tools
Risk-based optimization tools can also be used to monitor risk during ongoing trials. By combining the results of the simulations with actual trial data using machine learning, forecasts are continually improved and verified.
This information can then be used to determine where attention is needed and which actions are required to mitigate risk, reducing stress for clinical trial supply teams.
When should you use risk-based clinical supply optimization?
Risk-based optimization tools are suitable for use:
- For all trials, including simple and complex trials, across indications
- In planning Phase 2 and Phase 3 trials
- For setting up supply strategy and IRT 6 months from study start
- For assisting with protocol design in order to ensure supply-friendliness
- For ongoing monitoring of trial demand
Conclusion
Forecasting and risk-based optimization solutions are not mutually exclusive. The two approaches to predicting demand are complementary, and each has their advantages during different times in the study lifecycle.
Being able to transition smoothly and at the appropriate time from forecasting to risk-based optimization is important. For this reason, it’s considered best practice to use one solution for both forecasting and optimization.
Risk-based management in clinical trials is a growing trend due to the increasing complexity and globalization of the trial supply chain. A supply optimization solution is a necessary component of any future-facing risk-based approach to clinical trial supply.
About the N-SIDE Supply App
Currently, the only available solution that adds risk-based optimization to clinical supply forecasting is the N-SIDE Supply App.
The Supply App has been used to optimize over 10,000 trials of all sizes and complexity, providing 20-50% overall cost savings and 20-60% drug waste reduction while ensuring no missed dispensing.
Because it offers proactive risk management capabilities, the Supply App also reduces stress for the clinical trial supply team throughout the trial lifecycle.
How can we ensure reliability in a clinical supply forecasting system? Learn more in the free guide: Simplicity vs. Reliability in Clinical Supply Forecasting.





Other resources you might find intersting

Article
Forecasting and optimization in clinical trials
Learn about the latest advances in clinical trial forecasting and optimization. Understand how technology can help you overcome challenges you face in your clinical trial supply management.
Read now
Webinar
Understanding the benefits of risk-based optimization on clinical trial supply planning
During this webinar, you will learn how to identify the differences between risk-based optimization and forecasting for clinical supply chain and manufacturing planning and much more!
Watch the replaySupply App
The N-SIDE Supply App is the only solution that adds a risk-based optimization approach to clinical supply forecasting.
Make data-driven decisions about overage, packaging, sourcing, IRT setup, depot shipments, and more, all while measuring the precise impact of these decisions on patient service level and budgets.





