Achieving Sustainability Through Clinical Supply Innovation


We’re at an inflection point for sustainability in clinical trials. As challenges continue to mount, pharma companies must innovate or risk falling behind the curve.
We recently hosted a webinar discussing sustainability in clinical supply. This article contains a high-level summary of some key discussion points from the webinar. For a deeper dive that includes detailed case studies, watch the webinar recording.
What’s impacting sustainability in clinical trial supply?
Sustainability within the clinical trial supply chain is reliant on three main factors: emissions, ethics, and resource management. Let’s explore some of the key components and trends driving change in these three areas.
Emissions
From the extraction of raw materials to the destruction of expired or wasted drugs, the clinical trial supply chain produces greenhouse gas emissions at almost all stages. While you might think clinical research plays only a small role in driving climate change, the collective impact is quite large. According to a 2021 study, global clinical research accounts for 27.5 million tons of greenhouse gas emissions per year; for reference, that is more than the entire country of Denmark.
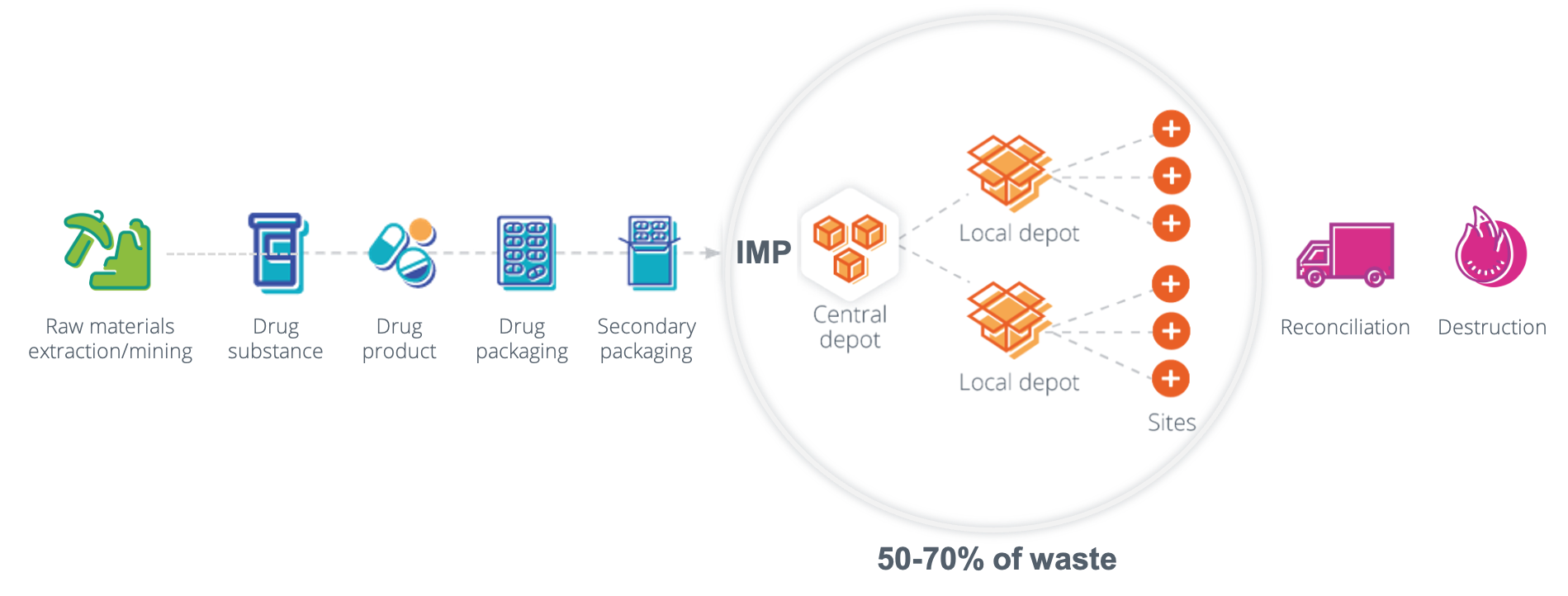
As a result, many pharma companies have announced goals to reduce their greenhouse gas emissions and lower their carbon footprint. Below are a few notable highlights:
- AstraZeneca aims to be carbon negative across the entire value chain by 2030
- Johnson & Johnson is striving to achieve net zero carbon emissions across the value chain by 2045
- GSK aims to have a net zero impact on climate and a net positive impact on nature across the value chain by 2030
- Pfizer aims to achieve net zero carbon emissions by 2040
- Lundbeck aims to achieve net zero carbon emissions by 2050
So, how are these companies actually moving forward with reducing emissions? The clinical trial supply chain can create emissions at any point along the supply chain (extraction, transportation, the power used in manufacturing, etc.), so there are a multitude of potential areas for reduction. By using mathematically driven machine learning and artificial intelligence techniques, biopharma companies can continue to optimize their clinical supply needs, shipping frequencies, and production capabilities, which can all help them achieve their sustainability targets.
Through extensive research, we at N-SIDE found that in clinical trials, 50-70% of drugs are wasted; this aligns with the waste level found by McKinsey & Co in a separate study. This means that up to 70% of all upstream emissions from extraction, manufacturing, and transportation result in no patient benefit. It is evident that there is plenty of opportunity for innovation within the current clinical supply processes to help companies achieve their sustainability targets.
Ethics
The term “sustainability” doesn’t only refer to emissions or the environment. In a bigger sense, the goal is to sustain the global healthcare system, ensuring patients receive their treatments on time and efficiently.
One major question: How does one approach patient treatment if there is a shortage in a comparator? The use of commercialized drugs in both clinical trials and day-to-day treatment for patients causes an ethical dilemma when these drugs fall into a shortage.
As of this writing, there are 149 drugs in shortage according to the FDA. This problem is especially pronounced in oncology, where 28% of phase III trials are using a drug that’s actively in shortage.
When a trial relies on a drug that is scarce, excessive waste will not only be expensive or inconvenient — it could also be unethical, or potentially deadly.
Resource management
At the center of sustainability is resource management. This tends to be the main headline in regards to how people view the term “sustainability.” Biopharma companies compete for the same finite resources in order to produce various drugs across many therapeutic areas. With finite resources, such as platinum, being crucial inputs for chemotherapies, it is imperative to maximize treatment output with minimal waste.
The semiconductor supply chain can also offer insight into the potential impact of shortages. In the semiconductor industry, the lack of forecasting and optimization technology has led to major disruption. Just as the lack of semiconductors impacted a multitude of industries (ex: cars, computers, cell phones, etc.) the lack of finite materials could have a similar impact on the pharmaceutical industry.
In order to prevent a total clinical supply failure, similar to the semiconductor industry, the next decades' challenges in the clinical supply industry stem from 4 factors:
- Raw materials shortage
- Manufacturers and CMOs shortage
- Increased competition for the same resources
- Finite investing capital
Precisely how these resources are managed will determine the sustainability of clinical trials in the future.
What can we do to improve sustainability in clinical supply?
Improving clinical sustainability can be done through various outlets, however, there is no easy way to stop all activities and reset all operations. Patients will always need to be treated and the business never has the time or capability to cease.
In order to improve the sustainability of a clinical trial, the most effective strategy is to produce a digital twin of the trial. A digital twin is a virtual model of the trial that can be used to test, simulate, and iterate changes to the trial. By doing this, companies can easily manipulate different components of their clinical trial, both before or during, without impacting the actual trial.
With a detailed digital twin, it becomes possible to quantify and optimize all aspects of the trial using simulations. You can change one parameter, and see how each of the other parameters will be impacted. Different recruitment scenarios, IRT settings, or IMP release plans can be tested to proactively forecast the unpredictability in the trial.
This approach helps all parties in the clinical trial process have a seat at the table and speak a common language. Clinical operations teams can understand how their assumptions can impact the supply manager’s forecast and vice versa. By being able to test all possible outcomes of the trial, companies can more effectively work as one to efficiently treat their patients.
The main issue with sustainability implementation is the prospect of what goes into becoming a “sustainable company”. Typically, sustainability teams need to be made, new processes established, more money needs to be spent, etc. However, by simply optimizing the supply chain with the current team and operations, decision-makers will quickly see a positive impact. Remember, by simply lowering the current 50-70% waste that is typically seen in clinical trials, the upstream emissions can already be reduced without any changes in teams, operations, or hiring needs.
Optimization using digital twin technology can help the pharmaceutical industry reduce waste by testing the lowest amount of overage required to effectively manage your supply chain. By reducing waste using risk-based optimization, companies can improve sustainability by limiting emissions, preventing shortages, and strategically managing existing finite resources.
N-SIDE’s software and professional services enable pharma companies to do just this. The Supply App helps you make data-driven decisions to reduce waste by using risk-based optimization. Additionally, the Production App helps pharmaceutical companies manage and optimize their end-to-end manufacturing, packaging, and production needs.
In the full webinar, we demonstrate these capabilities via two case studies, including:
- A respiratory drug trial for COVID that required a comparator in shortage, in which waste was reduced by 32%
- An upcoming oncology trial with several unique challenges related to expensive biosimilars and scarce chemotherapies which resulted in the protocol being adjusted to reduce demand for the scarce drugs



About the Author
Jake Levine is a Solutions Engineer at N-SIDE. His experience in clinical supply expanded quickly by solving day-to-day supply challenges of biopharma companies. He is passionate about utilizing artificial intelligence and machine learning to improve the clinical supply industry and, ultimately, improve patients’ health. Jake received his Master’s Degree in Healthcare Systems Engineering from Lehigh University where he developed a strong interest in healthcare and biopharmaceutical market disruption, with a patient-centric approach
Jake Levine




Content you might also like

article
What is a clinical trial supply risk management plan?
When we talk about risk management in clinical trial supply, we’re usually referring to two types of risk: the risk that patients will miss dispensing and the risk of global drug shortage.
There are two types of clinical trial supply risk management plans: proactive and reactive.
read more
webinar
Designing a clinical trial: Tips and tricks to streamline your clinical supply chain
With pharma and biotechs’ ambitions to do more with less, the supply chain quickly becomes the center of attention in the attempt to supply trials faster, more efficiently, and with lower waste levels.
Discover how some often overlooked parameters can have a significant impact on IMP waste and supply risk.
watch on demand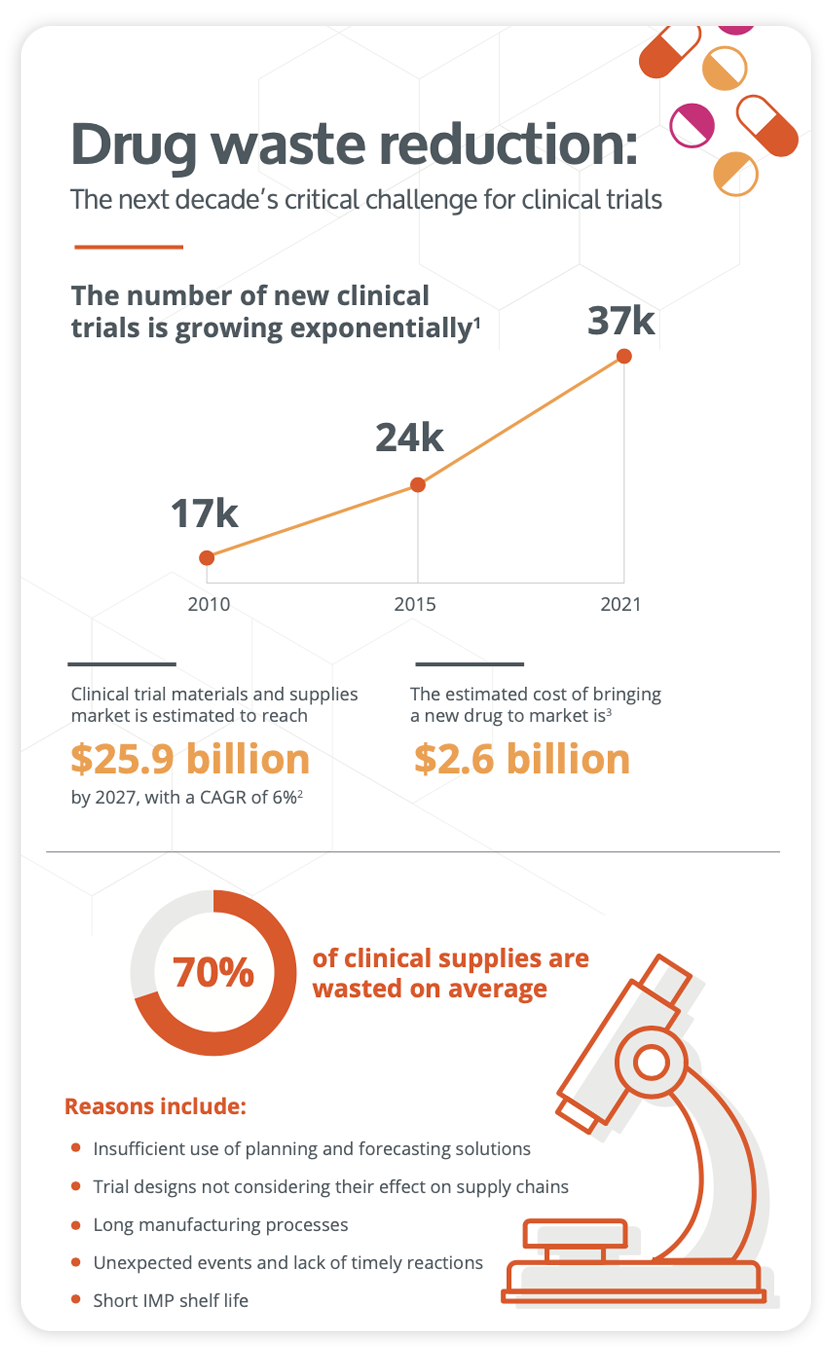
infographic
Curb drug waste and improve your clinical trial strategy
They are many factors that contribute to bottlenecks in the supply chain, including the increasing complexity of clinical trial designs, the shorter products shelf lives in innovative treatments, a severe shortage of manufacturing resources, etc.
See what clinical supply chain managers can do to curb these risks.
read more

