Curb drug waste and improve your clinical trial strategy


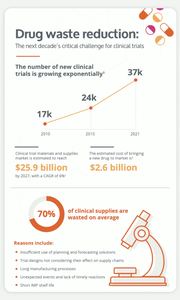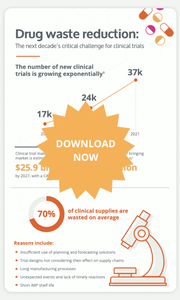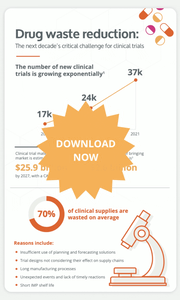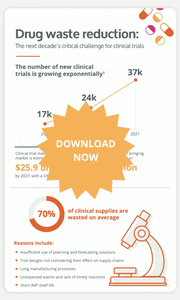
An average of 95% of clinical supply managers face some level of supply risk during their trials. Moreover, supply chain bottlenecks can generate 2 to 4 months of delays in the drug development timeline.
They are many factors that contribute to bottlenecks in the supply chain, including the increasing complexity of clinical trial designs, the shorter products shelf lives in innovative treatments, a severe shortage of manufacturing resources, etc.
The number of new clinical trials growing exponentially, long manufacturing processes and high levels of waste are all impacting the clinical trial supply chain management. It is undeniable that drug waste reduction will be the next decade's critical challenge for clinical trials. But what can clinical supply chain manager do to curb these risks?
One way to achieve this is to reduce your global level of waste. In turn, you can also discover how drug waste reduction can accelerate drug development timeline and help streamline your supply chains. Solutions like the N-SIDE exist to help you achieve better results and a less stressful clinical trial strategy management.
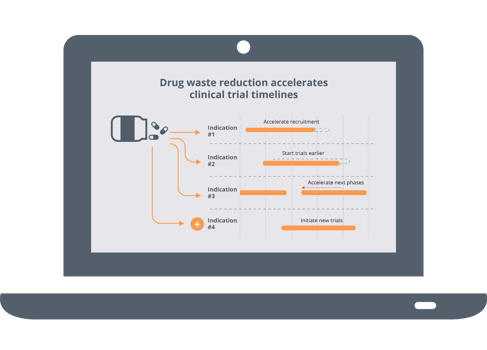
Download this infographic to learn about the different challenges you might face and how you can streamline your clinical trial strategy thanks to the help of our technology. Waste mitigation and all its associated rewards can indeed be achieved with N-SIDE's clinical supply solutions for the benefit of all stakeholders.





Other resources you might also like

Article
Take the guesswork out of clinical trial supply overage
With risk-based optimization, Clinical Supply Managers are able to manage their trials in a more relaxed and efficient way, without worrying about having enough overage.
Read now
White paper
Accelerating time to market through clinical supply and manufacturing optimization
Hear from Biotech industry experts how challenges in clinical trial supply and manufacturing can be overcome with innovative software solutions.
Download it now!
Webinar
Assessing the impact of assumptions on Clinical Supply Planning
During this webinar, you will learn that misused assumptions cause more than 20% of your drug waste and risk, what are the most relevant assumptions required for a robust forecast and the impact each assumption has on the supply chain forecasts and patients.
Watch now!





