Woops, there's no exact match to what you are looking for. But we selected relevant content that you might also be interested in:

N-SIDE introduces Lighthouse, innovative software to shape the future of worldwide clinical trial supply management
Lighthouse will double the amount of active clinical trials managed by N-SIDE to 1,400 within 3 years
Read more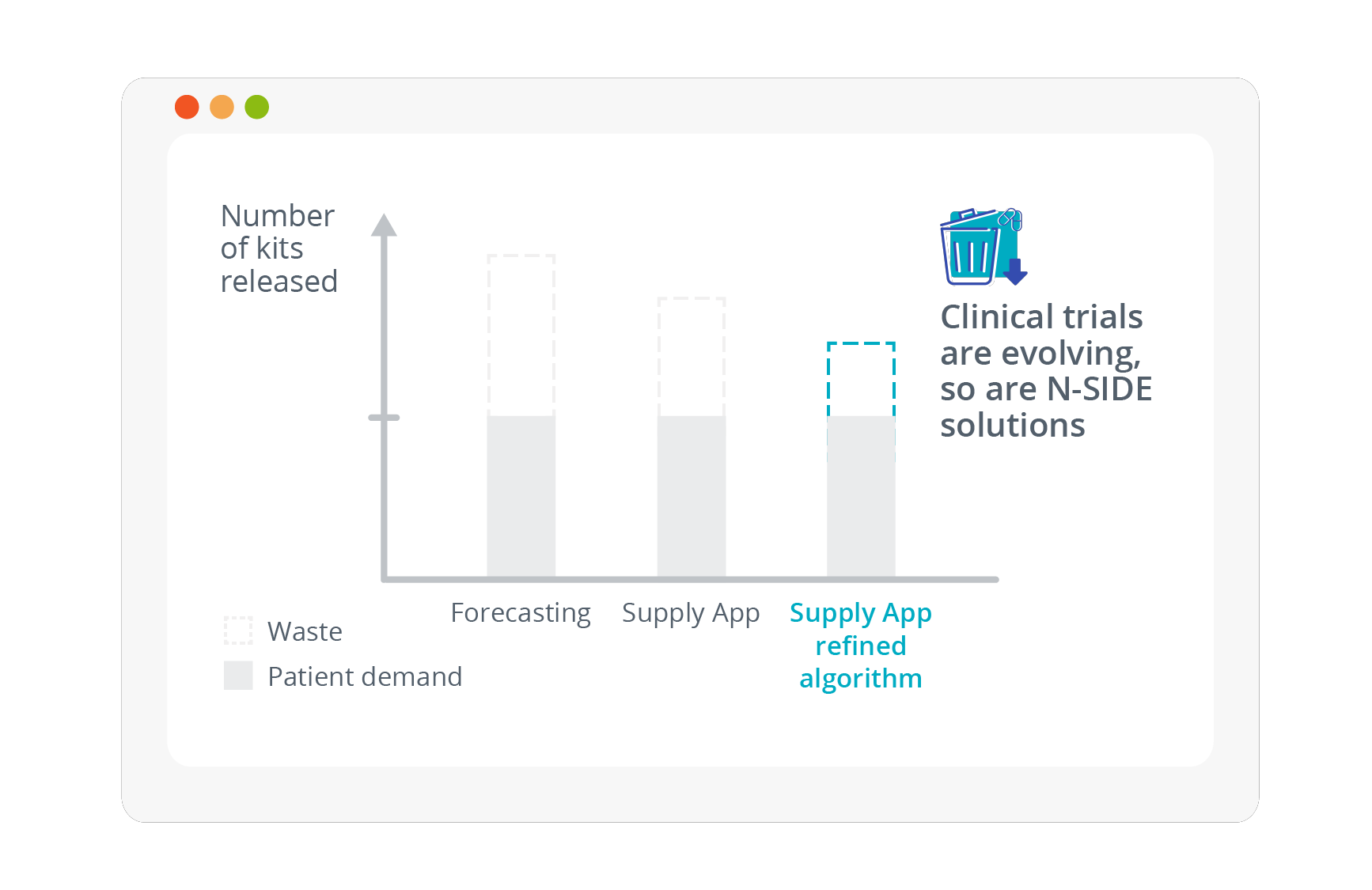
Decrease clinical trial supply chain waste even more with the N-SIDE Supply App refined algorithm
On average, between 55-75% of drugs are wasted in clinical trials. Overproduction has historically been accepted in clinical supply and manufacturing processes...
Read more
IRT optimization in clinical trials: achieving maximum efficiency
Clinical trial supply managers work hard to ensure trial protocols, supply plans, and resupply strategies are designed for maximum efficiency.
Read more
Curb drug waste and improve your clinical trial strategy
An average of 95% of clinical supply managers face some level of supply risk during their trials. Moreover, supply chain bottlenecks can generate 2 to 4 months...
Read more
Let's meet at our next conferences!
After a couple of quieter summer months, N-SIDE is headed to conferences again! Missed us last year or want to meet with us again? You're in luck! Find out which...
Read more
5 key considerations for clinical trial packaging and labeling
Designing the packaging and labeling for investigational new drugs (INDs) and investigational medicinal products (IMPs) can be a complex task — especially when...
Read more
The 3 biggest challenges in the clinical trial supply chain
Drug supply chain management is a balancing act. You need to ensure enough drug is available to meet patient demand and that supply is allocated where it’s most...
Read more
How to optimize your clinical trial manufacturing plan
Are you making the best possible decisions about your clinical trial manufacturing?
Read more
What is clinical supply chain software?
As clinical trials become more complex and expensive, it becomes even more important that they go smoothly from the start. Thankfully, clinical supply chain...
Read more

