The 3 biggest challenges in the clinical trial supply chain


Drug supply chain management is a balancing act. You need to ensure enough drug is available to meet patient demand and that supply is allocated where it’s most needed. Meanwhile, you have to minimize costs and timelines while also preparing for the unexpected.
Today, the three biggest challenges in the clinical trial supply chain are uncertainty, siloed decision-making, and supply bottlenecks.
But there is a solution: a resilient supply chain.
Built on a foundation of redundancy, flexibility, and a culture centered on communication and collaboration, a resilient supply chain helps you not only manage risk but positions you to manage risk better than competitors.
Here, we’ll explore each of the three major challenges and provide solutions that can make your clinical trial supply chain more resilient.
Challenge 1: Uncertainty
The clinical trial supply chain is built on uncertainty. Factors like recruitment speed, patient attrition, maximum tolerable doses, titrations, and patient attributes are inherently uncertain, making it difficult to operate the supply chain efficiently.
But there is also another kind of uncertainty caused by external factors like war, natural disasters, and pandemics. COVID-19, for example, showed us just how precarious the clinical trial supply chain can be.
It’s estimated that the pandemic slowed down, interrupted, or stopped some 80% of non-COVID-19 clinical trials: Communication lapsed, laboratories and warehouses closed, and teams lost access to equipment and resources. Lead times for manufacturing, packaging, labeling, and distribution became much longer and threatened trial success. Meanwhile, safety issues impacted recruitment in more than two-thirds of trials, causing delays that led to high drug waste.
Are you prepared for the next disruption? Clinical trial supply chains must be agile in order to quickly and effectively respond to uncertainty. One way to become more agile is by using an end-to-end clinical supply chain solution such as the N-SIDE solutions.
Combining solutions for clinical trial manufacturing and supply optimization, the N-SIDE solutions can enable pharmaceutical companies to:
- Increase visibility and transparency throughout the entire supply chain
- Use scenario planning to help prepare for disruptive events
- Quantify and proactively mitigate risk of drug shortages
- Continuously streamline your manufacturing plan with machine learning that adjusts to real-time events
- Use monitoring dashboards that illuminate the limits of your current planning
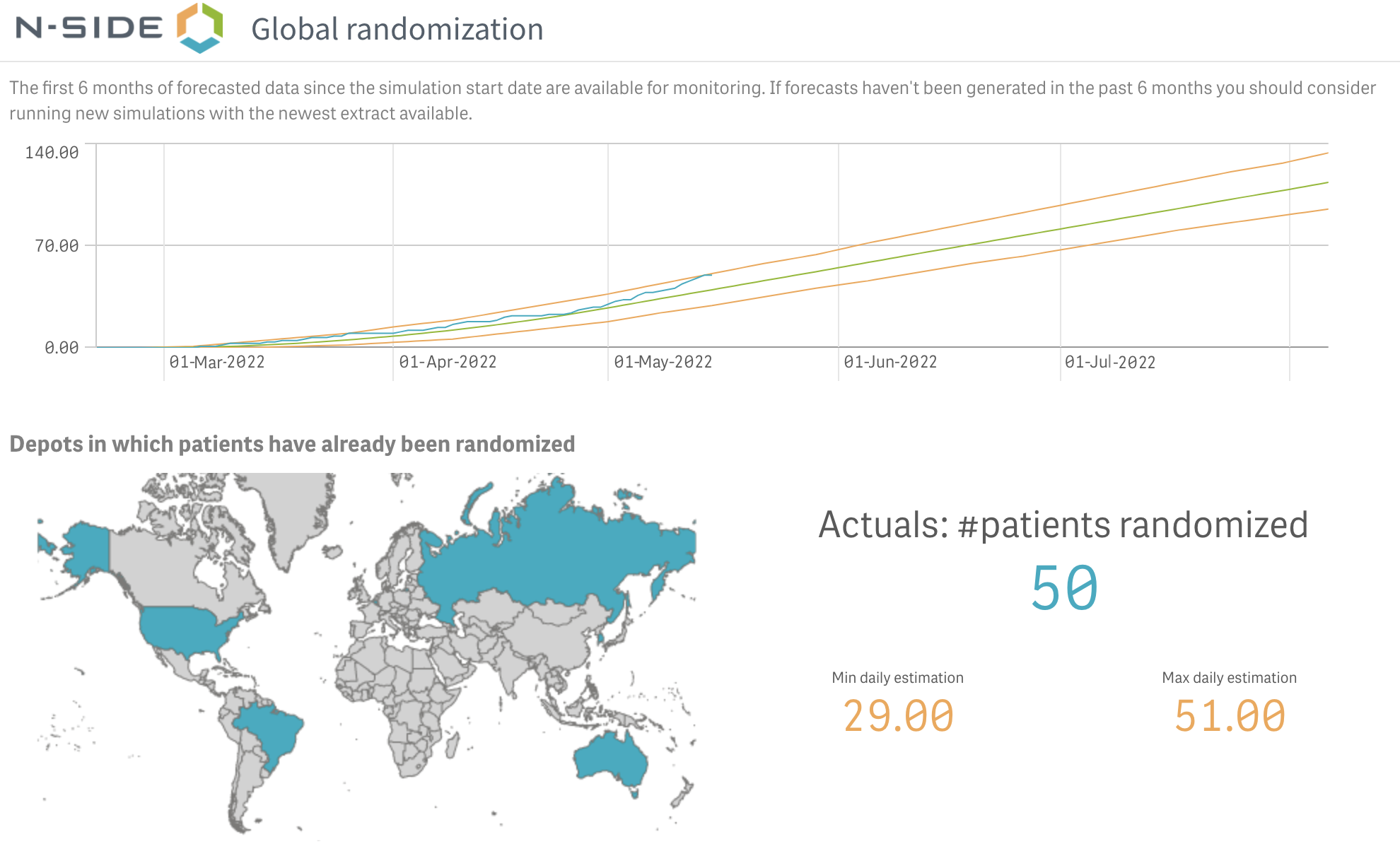 N-SIDE solutions - Monitoring dashboard
N-SIDE solutions - Monitoring dashboard
Challenge 2: Siloed decision-making
To achieve an efficient end-to-end clinical trial supply chain process, it’s important to share reliable, up-to-date data between departments and assess the impacts of decisions made in one department on each of the others. However, these decisions are often made by different teams with different objectives in different systems, making communication difficult.
When you don’t have a global view of the clinical trial supply chain, you risk miscommunication, missing data, and a lack of centralized decision-making. You’re forced to assess challenges and come up with solutions independently at every level of the supply chain — without knowing if those solutions make sense for the trial or program overall.
This typically leads to overproduction, high drug waste, increased costs, and long delays.
To build a resilient supply chain, information must be shared upstream and downstream in a timely and accessible way. For example, supply and CMC need to be informed as soon as possible of changes to trial design or timelines. Supply and manufacturing should even be included in the decision-making process for trial and protocol design, given the deep connection between these activities.
At the same time, manufacturing data about lot size, allocation, stability, and other manufacturing constraints should be shared downstream to help supply teams optimize their supply strategies.
Are you willing to take on the risks of siloed decision-making? With clinical trial supply chain solutions like N-SIDE’s Supply and Production Apps, you can reduce risk by helping teams:
- Make proactive data-driven decisions to drive cross-team communication
- Ensure immediate data sharing and visibility throughout the trial lifecycle
- Assess the impact of any decision on the global supply chain
- Take a global view that breaks down data silos in the planning stage
- Account for constraints at every level
Challenge 3: Supply bottlenecks
Today more than ever, manufacturing resources are scarce and drugs are expensive. But did you know that in an average trial, 55-75% of drugs are wasted?
Not only does drug waste increase your costs; it also leads to supply bottlenecks that slow down clinical programs. Unused supply delays the initiation of other trials that could have benefitted from having access to it and prevents you from bringing your drug to market on schedule.
Overproduction may have been a long-accepted part of the clinical trial process, but is it really sustainable in the long run? The global shortage of manufacturers, the complexity of producing biologic drugs, and the high price of raw materials all point toward overproduction becoming a less viable strategy as time goes on.
Now, risk-based clinical trial supply optimization can help you pinpoint precise levels of drug overage required to cover the risk of missed dispensing while optimizing decisions to keep it low and eliminate the supply bottlenecks caused by overproduction.
Building a resilient clinical trial supply chain
Some challenges and disruptions to the clinical trial supply chain are unavoidable (like a pandemic), but a resilient supply chain will help you stay agile and be prepared for whatever may come.
Optimize your entire clinical trial supply chain by taking a global view and making data-driven decisions at every decision-making level. The N-SIDE solutions can support you in becoming more agile and resilient, so you can get drugs to the patients that need them while decreasing risk, increasing efficiency, and reducing waste.
Learn more in this whitepaper: Reduce the stress of clinical supply chain planning by taking the global view.



About the Author
Amaury is N-SIDE's Strategy Advisor for clinical supply chain solutions. Within his 9 years in the industry, Amaury’s objective has been to revolutionise planning and systems to make clinical supply chain more efficient, more ethical, less wasteful and more patient-centric.
Amaury Jeandrain



Article
Forecasting and optimization in clinical trials
Learn about the latest advances in clinical trial forecasting and optimization. Understand how technology can help you overcome challenges you face in your clinical trial supply management.
Read now
Whitepaper
Understanding the differences between Simplicity and Reliability in Clinical Supply Forecasting
Forecasting tools don’t include several important factors, which can lead to less reliable results. Inaccurate forecasts threaten supply continuity, can lead to large amounts of drug waste and, in some cases, be risks to patient health.
Download now
SOLUTION
The N-SIDE solutions
This end-to-end platform powers the clinical supply chain, supporting strategic decision making, operations, and enhanced monitoring at both the trial and program levels.
DISCOVER NOW

