Accurately forecasting supply for clinical trials has always been one of the biggest challenges for study managers? Now, new sources of uncertainty and complexity are making this problem even more difficult to solve.
Clinical supply forecasting tools claim to offer a solution to this problem, and in recent years, these digital solutions have become simpler and more automated. Unfortunately, this simplicity often comes at the expense of accuracy.
Inaccurate forecasts threaten supply continuity and can lead to large amounts of drug waste or, conversely, risks to patient health. For this reason, accurate forecasting is an ethical imperative as much as a commercial one.
In this whitepaper, we’ll explore the challenges that are making it increasingly difficult to forecast clinical trial supply, and look at a solution that has been proven to help clinical trial managers succeed in bringing accuracy to their planning.
Sources of clinical trial supply uncertainty
While clinical trial designs vary widely, uncertainties in planning for the supply chain are rooted in a few key areas.
Uncertainty surrounding patients
Trial managers don’t know when or where patients will arrive, in which treatment arm, how they will react to the treatment, and whether they’ll tolerate the dose. There is also no certain way to predict titrations, secondary effects, or physical characteristics such as weight and body surface area. This makes the demand inherently unpredictable.
Uncertainty surrounding timelines
Due to strict regulations in clinical trials (GxP, customs, country-specific regulations) and their drug supply, it can take months to plan and arrange manufacturing, releases, or shipments of trial drugs.
Add the fact that shelf lives are generally very short, and it becomes extremely challenging to accurately plan for the right quantities to be produced and distributed.
Uncertainty surrounding team alignment
The clinical supply chain needs to be set up based on decisions made by multiple teams that have different targets and objectives. The clinical team might define the trial protocol to optimize retention and recruitment, while another team designs the trial kit with patient centricity in mind, and yet another sets up the IRT. It is important for the trial supply manager to be able to align all of these decisions and constraints in order to understand the impact on supply.
Each of these elements — patients, timelines, and team alignment — make it difficult to accurately forecast the delivery and dispensing of medication to the right patients in the right places at the right times.
Trial complexity is increasing
While uncertainty has always been a central challenge of the clinical trial supply chain, the level of uncertainty is increasing as trials become more and more complex.
New therapeutics require more complex trials than their predecessors. Biologics, for example, have shorter shelf lives and more complex manufacturing processes than conventional drugs, while cell and gene therapies and new vaccines, such as those using mRNA technology, often require specialized transport and storage.
Newer drugs also often target rarer diseases and therefore smaller patient populations, which makes recruitment difficult. That in turn adds complexity, leading to an increased number of countries and sites to be initiated, higher uncertainty regarding timelines, and reduced efficiency in the supply chain.
Meanwhile, with the COVID pandemic, there was a rise of decentralized, direct to patient, trials. Study drugs in these trials need to be delivered directly to patients homes. This required a new way of managing trial supply.
In addition, there has been an increase in innovative trial designs: Adaptive, umbrella or basket trials aim at including multiple different indications or populations within the same protocol, and tend to be subject to frequent protocol amendments. Dose ranging trials also test multiple dose levels without knowing in advance which ones will be successful and provided to a wider patient population.
All of these complexities increase uncertainty, which has to be taken into consideration when running supply forecasts. Therefore, modern clinical supply forecasting tools must be accurate, flexible, and able to incorporate as many constraints, details and sources of uncertainty as possible.
Simple demand forecast models look straightforward but could create unwelcome surprises
Unfortunately, many clinical supply solutions and methodologies take the opposite approach, striving for simplicity rather than accuracy. Many trial design models need to be simplified (or users are encouraged to simplify them) in order to save time or match the solution’s capabilities. Also, for the sake of automation or per system limitations, uncertainties are hardly considered.
What is a clinical supply forecasting tool?
These digital solutions use simple algorithms that estimate how many clinical trial kits are needed based on the trial design, the number of patients, and their assumed treatment dose, to which an estimate of overage or drug wastage is manually added. There are many different forecasting tools on the market today.
Many sources of information are therefore overlooked in the forecast. While keeping things simple may give a sense of security to the user, this can have a huge impact on patient service and drug supply. While not every forecasting solution works the same way, frequently overlooked factors include:
- The accuracy and variability of recruitment and patient titration (timing, location)
- The accuracy in representing the IRT/RTSM algorithm
- Drug expiry and expiry replacements
- Expiry extensions
- Demand variability at the depots
- Risks
Say you’re forecasting a trial in Brazil with a protocol that includes titrations and weight-based dispensing. Unfortunately, your forecasting model does not take this into consideration when planning for packaging quantities. Based on its calculations, you should send 100 kits to Brazil. But is that enough? How many patients will those 100 kits cover? What if patient recruitment is slightly higher than expected? What if patient weights and dose level are slightly higher than expected? When would we be at risk?
Even small shifts in any of these factors, which are not considered by most forecasting tools, can result in demand that can vastly exceed — perhaps even double — the initial estimate, leading to increased risk of missed doses.
Most forecasting solutions also don’t attempt to model supply chain complexity. This can be problematic, because it means their forecasts don’t take into consideration the impact of expiry replacements, lead times, and rules for sites and depots to be resupplied. If they’re not aligned, depot and site stocks can be depleted much faster than production can fill them.
Obviously, increased patient risk is not an acceptable outcome. That’s why users generally complement their forecasts with a manually estimated overage and conservative demands and buffers in order to cover potential inaccuracies. Unfortunately, because there is so much uncertainty, these overages can often be too high, creating unnecessary drug waste (and leading to increased costs) or too low, leading to risk.
More reliable forecasts enable optimization
Achieving more reliable forecasts requires accurate assessments of complexity and uncertainty.
While no model can tell you exactly what will happen in the future, more robust models can incorporate as many details as possible in order to assess uncertainty. Simulations would then factor in any variability inherent to patients’ recruitment, titrations, treatment length, or even temperature excursions, to generate thousands of possible futures, with best and worst case demand models provided as an output. This information can then be used to not just forecast supply, but optimize it in order to minimize drug waste while still planning the right amount of drug at the right place to cover even a worst case.
In this scenario, overage is not a manual estimate, but a product of the optimization algorithm. With an accurate model, the relative amount of overage will exactly match the complexity and uncertainty of the trial, covering risks while avoiding unnecessary waste, thereby freeing up production resources and accelerating timelines.
A more reliable forecast should also inform the supply manager exactly what’s covered in the calculations, rather than simply producing an average demand. Reliable forecasts should be able to provide a threshold within which the model is confident that the forecasted strategy is valid.
Reliable forecasts should also be connected to real-time trial data. Using this data, the forecasting tool should show the user when real-time factors exceed the threshold of its initial forecast, so the forecast can be updated accordingly.
Our solution:
the N-SIDE Supply App
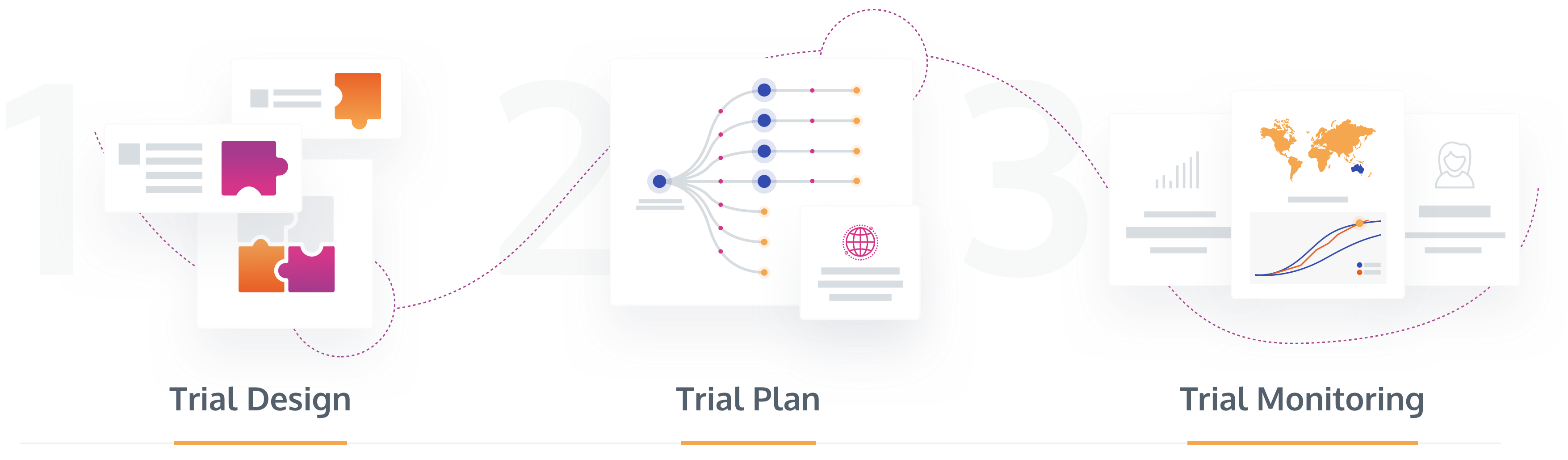
N-SIDE offers the only clinical trial supply optimization solution capable of accurately modelling all trial designs, while correctly assessing the trial uncertainty and providing a reliable forecast and supply chain strategy that minimizes risk and waste. The Supply App supports all stages of the clinical trial lifecycle for increased reliability, including designing, planning and monitoring.






