The challenges of a cell & gene therapy supply chain


Designing high-performance, risk-controlled, and scalable cell & gene supply chains with a digital twin
[PART 1] - The challenges of a cell & gene therapy supply chain
Cell & gene therapy (CGT) is an umbrella term covering medicinal technologies that rely on:
- the injection of living cells into a patient, and/or
- the amelioration of the genetic material expressed by the patients.
Over the past decades, CGT technology has rippled throughout the pharmaceutical world and attracted much attention. Thousands of therapies are being developed and about a hundred have already been approved. 16 of the world’s largest 20 bio-pharma companies have CGT assets in their product portfolios.
The goal of CGT is generally to restore or elicit some biological activity. To this end, a wide variety of technologies and mechanisms of action are leveraged, for example:
- Immunotherapy: T cells, which are cells of the immune system, are recovered from the patient’s blood and infused back after they have been genetically modified to express engineered receptors called chimeric antigen receptors (CARs), allowing CAR-T cells to detect cancer cells that would normally go undetected. This allows for the targeted treatment of a variety of diseases such as leukemia, myelomia, and colorectal cancers.
- Genome augmentation with viral vectors: Luxturna (Novartis) targets visually impaired patients and uses a virus to carry a functional copy of a defective gene into the retinal epithelium of the patient, restoring the normal production of the missing protein involved in vision.
- Tissue repair: MACI (Vericel) heals knee cartilage damage by extracting and multiplying the cells of a healthy piece of cartilage obtained from a patient and then re-implanting them into the patient within a collagen matrix. In Novadip’s process, stem cells from the patient obtained from fatty tissue are cultured in vitro to become a biomaterial consisting of bone-forming cells.
While these therapies create a whole new treatment paradigm, their characteristics make development and commercialization particularly challenging in many regards. For example, the reimbursement of these exorbitantly expensive (up to several million $) but potentially curative products requires innovative financial approaches. Likewise, it is fair to say cell and gene therapies are more difficult to manage compared to small molecules or biologics.
For cell therapies (see Exhibit 1), this implies that some human cellular material must first be recovered as the starting material, either from a healthy donor (allogeneic) or from the patients themselves (autologous). Living cells are extremely fragile and must be processed within strict time and preservation temperature boundaries. This extra supply chain step is notoriously difficult to operate as it requires strong coordination with the recovery centers and the logistics providers.
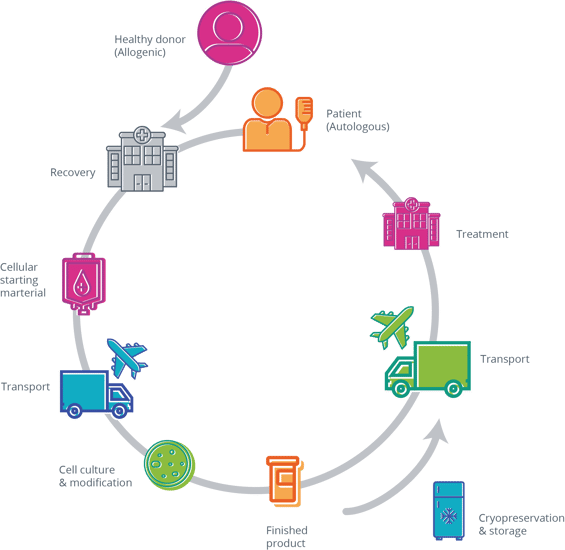
[Exhibit 1 CGT] supply chains
For autologous therapies in particular, the whole supply chain flow is triggered by the identification of a new patient, often from a small population of eligible patients. Consequently, CGT manufacturers must be able to respond to unplanned demand. Moreover, since each batch is specifically produced for a single patient, it is useless to scale up the manufacturing process by increasing the batch size beyond the quantities required for one treatment. Instead, pharmaceutical companies scale out by deploying multiple smaller manufacturing units that can be exploited to deliver multiple batches concomitantly.
The duration of the manufacturing process itself is long (typically a few days to a few weeks), often variable, and must be performed in strict aseptic conditions. The yield is uncertain, partly due to the intrinsic nature of the cellular starting material. The quality controls are complex and time-consuming too.
Next, the finished product must be distributed back to the patient. This can be done either at refrigerated temperatures (typically 2-8°C) or at cryogenic temperature (i.e., <-150°C). Refrigerated temperatures are associated with extremely short shelf-life (in hours or days), necessitating strong coordination between the sponsor, the logistics provider, and the healthcare professionals. Sometimes this can be alleviated by manufacturing the CGT close to or at the point of care rather than centrally. This however creates extra challenges to maintain consistency in the network of manufacturers.
Cryogenic preservation requires a well-controlled freezing process and the ability to maintain extremely low temperatures throughout the supply chain, including at the point of care. However, the cryogenic infrastructure needs to catch up to more established refrigerated and frozen product distribution and storage. Typically, cryogenic products are transported in non-disposable tanks filled with liquid nitrogen. This requires specific processes to prepare the tanks for shipping, to maintain and verify their performance, and to get them back after delivery.
The high product price tag also imposes that it is distributed only in response to a well-identified need. This starkly contrasts with the classical distribution process, where a point of care anticipatively builds inventory at their pharmacy.
In summary, this complexified supply chain results in 9 key challenges depicted in Exhibit 2.
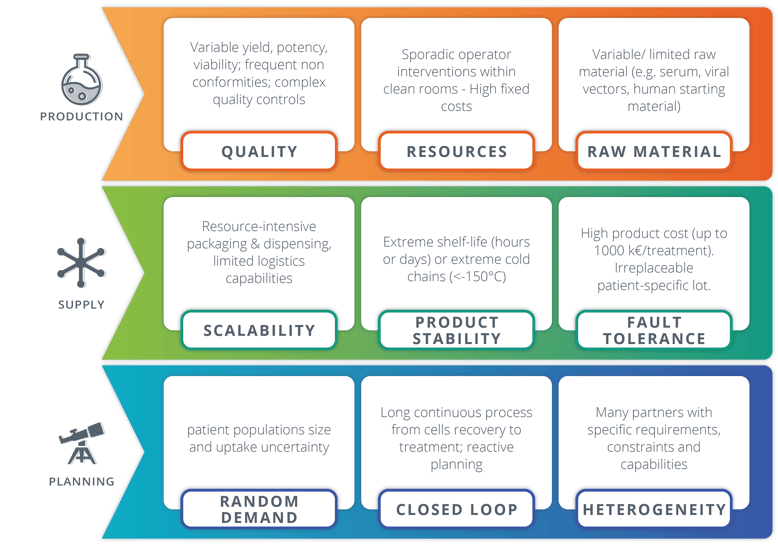 [Exhibit 2] Nine key challenges of CGT supply chains
[Exhibit 2] Nine key challenges of CGT supply chains
Beyond drug discovery, innovation entails bringing CGT to patients in need, both during development and after commercialization. As more CGT move from research to clinical development and commercialization, capacity planning and supply chain strategies are a rising concern. Strikingly, research shows that 20% of cancer patients who are eligible for CAR-T therapies pass away while waiting for a manufacturing slot.
In a next article, we’ll explore how digital twin and stochastic modeling can be powerful tools for optimizing the supply chains of cell and gene therapies, providing supply chain managers with valuable insights into operational performance and helping them make early informed decisions.



About the Author
Philippe has been helping pharma and biotech companies create and operate their supply chains for more than 20 years. Before joining N-SIDE as Strategic Project Leader, he directed the supply chain of a cell therapy company for 8 years and gained a firsthand experience of the specific challenges of this technology. He obtained a PhD in Bioengineeing, by creating a computational model of protein/membrane interactions. He also holds a certificate in Management for Biomedical Industry Executive.
Philippe Ducarme




Other content for you

article
Explained: Forecasting and Optimization in Clinical Trials
Growing challenges related to clinical trial management have renewed interest in forecasting and optimization solutions.
Many pharmaceutical companies and contract research organizations (CROs) are examining their existing forecasting and optimization processes and searching for more effective solutions.
Read more
blog post
The 3 biggest challenges in the clinical trial supply chai
Drug supply chain management is a balancing act. You need to ensure enough drug is available to meet patient demand and that supply is allocated where it’s most needed. Meanwhile, you have to minimize costs and timelines while also preparing for the unexpected.
Today, the three biggest challenges in the clinical trial supply chain are uncertainty, siloed decision-making, and supply bottlenecks.
read more
white paper
End-to-end clinical supply chain optimization helps bring drugs to market faster
With a growing global population, speeding up drug development timelines is of paramount importance for all pharmaceutical and biotech companies. Thanks to innovative software solutions it’s now possible to make better decisions at each stage of the clinical supply chain.
download now
