The challenges of a cell & gene therapy supply chain - part 3


Designing high-performance, risk-controlled, and scalable cell & gene supply chains with a digital twin
[PART 3] - Optimal manufacturing capacity
In the preceding articles of this series [part 1 - part 2], we described how the supply chains of cell & gene therapies are particularly complex and why digital twins are effective tools for designing these supply chains.
Amongst the myriad of questions gnawing at the cell & gene therapy developers, one is to build the manufacturing capacity adequately.
For autologous therapies in particular, where the starting material is recovered from the patient who later receives the treatment, the number of identical manufacturing units determines the ability to meet demand:
- Scaling up is not effective, given that each batch is patient-specific. Therefore the only way to achieve a higher capacity is to run several smaller production units;
- The starting material and finished products can hardly be stored and this prevents leveling out unpredictable demand. Therefore the capacity in place completely determines how many patients can be served.
So, how many manufacturing units exactly should be available to treat all patients quickly enough?
Intuitively, running the manufacturing plant at 100% of its capacity seems optimal. However, in this configuration, any accidental delay in the manufacturing would create a waiting queue in front of the production line since all manufacturing units are already being used. By the same token, as there is no extra capacity to clear this backlog, the queue and the total turnaround time will continue to grow over time as adverse events pile up. At some point, the manufacturer will be forced to decline some manufacturing requests as the turnaround time becomes unacceptable.
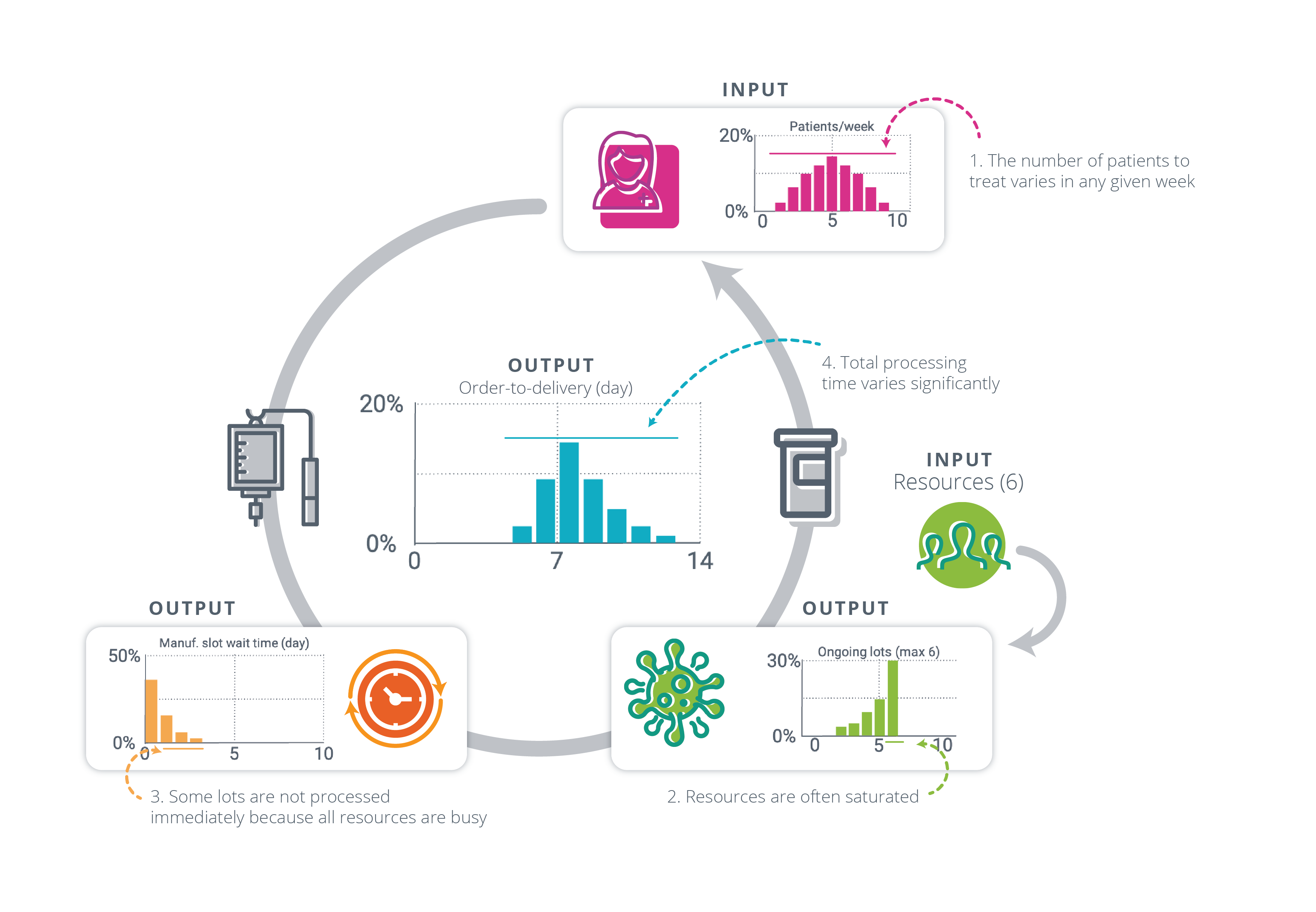
Problems may persist even when the manufacturing capacity exceeds the average demand. For example, with a number of patients to treat distributed around an average of 5 patients/week, even a manufacturing capacity of 6 lots/weeks may become saturated from time to time, which creates a waiting queue and increases the average turnaround time (Exhibit 1).
[Exhibit 1] Stochastic models use distribution as inputs/outputs
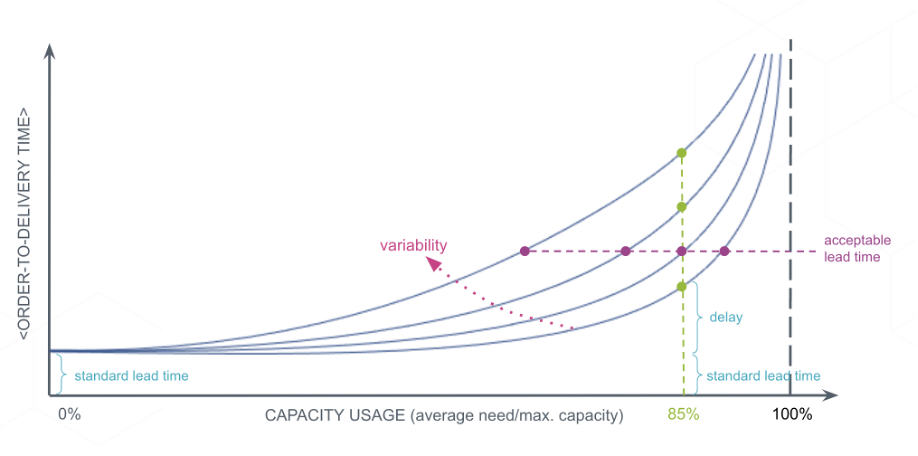
More generally, the total turnaround time can be computed as a function of the manufacturing capacity used (See Exhibit 2).
The exact shape of the curve, which can be determined by a digital twin, depends on the variability of the duration of the manufacturing process, with more variable processes producing longer turnaround times. Once the curve is known, it becomes possible to determine the optimal capacity that must be put in place to respond to the demand within an acceptable turnaround time.
[Exhibit 2] Average order-to-delivery time as result of capacity usage and variability
If we want to increase the number of patients treated concomitantly within a maximum turnaround time, there are multiple options, for example:
- Decrease the manufacturing lead time (e.g. faster cell growth)
- Decrease the manufacturing lead time variability (e.g. well-defined starting material)
- Decrease the demand variability (e.g. centralized manufacturing)
- Increase the number of manufacturing resources (e.g. night work)
Under any of these hypotheses, the digital twin will be able to determine the resulting performance in terms of cost, risk of failure, waste, or whichever performance indicator is relevant to compare the strategies and objectively select the best approach.
More complex processes with discrete events can also be taken into account. For example, one may consider that a clean room is made unavailable while being requalified, after x runs or by a fixed date, whichever comes first, and that the duration of requalification is also a random variable.
Finally, any creative solution can also be tested. For example, would it help to set up a pool of operators who can rapidly commute between manufacturing locations? And this is only a token of what digital twins can achieve as there is no limitation on the number of processes that can be interconnected.
As we can see, an effective supply chain design can only be designed by taking into account its variability. This is precisely what stochastic modeling and digital twins aim for.



About the Author
Philippe has been helping pharma and biotech companies create and operate their supply chains for more than 20 years. Before joining N-SIDE as Strategic Project Leader, he directed the supply chain of a cell therapy company for 8 years and gained a firsthand experience of the specific challenges of this technology. He obtained a PhD in Bioengineeing, by creating a computational model of protein/membrane interactions. He also holds a certificate in Management for Biomedical Industry Executive.
Philippe Ducarme




Other content for you

article
Explained: Forecasting and Optimization in Clinical Trials
Growing challenges related to clinical trial management have renewed interest in forecasting and optimization solutions.
Many pharmaceutical companies and contract research organizations (CROs) are examining their existing forecasting and optimization processes and searching for more effective solutions.
Read more
blog post
The 3 biggest challenges in the clinical trial supply chai
Drug supply chain management is a balancing act. You need to ensure enough drug is available to meet patient demand and that supply is allocated where it’s most needed. Meanwhile, you have to minimize costs and timelines while also preparing for the unexpected.
Today, the three biggest challenges in the clinical trial supply chain are uncertainty, siloed decision-making, and supply bottlenecks.
read more
white paper
End-to-end clinical supply chain optimization helps bring drugs to market faster
With a growing global population, speeding up drug development timelines is of paramount importance for all pharmaceutical and biotech companies. Thanks to innovative software solutions it’s now possible to make better decisions at each stage of the clinical supply chain.
download now