Saving a trial from running into drug shortage


 Mégane Noirfalise
Mégane Noirfalise
Although our solutions are ideally used proactively before the start of a trial to ensure a smooth sailing through the trial's lifecycle, we are sometimes called after a trial starts to help solve all sort of predicaments, including drug shortages.
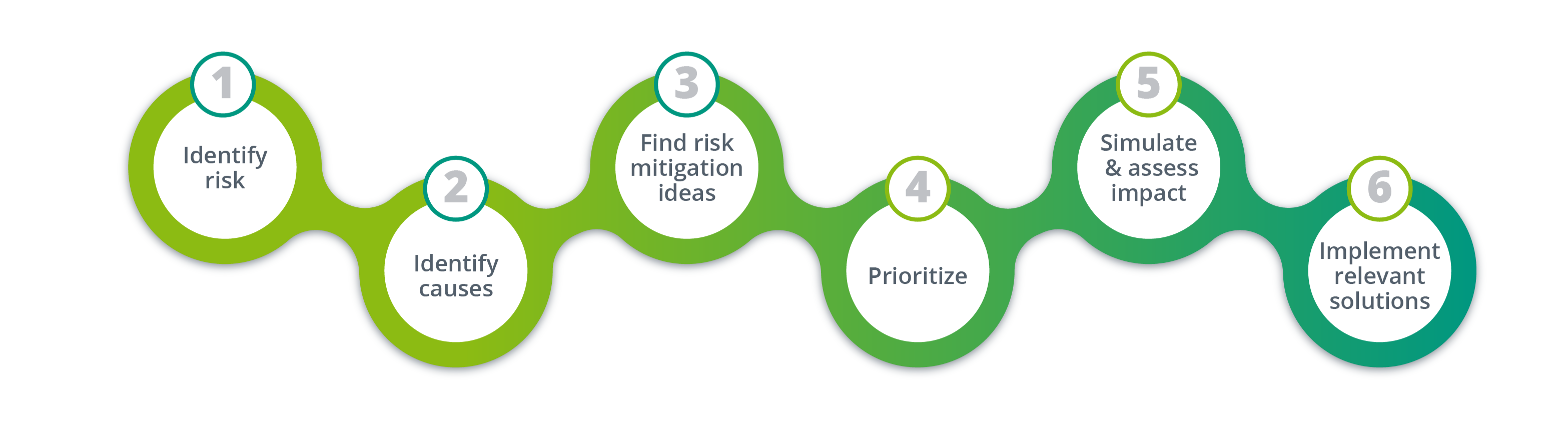
We recently were called by a small biopharma company 6 months after the initiation of their trial because they were about to run into a drug shortage situation. In this case, we found ourself in a reactive risk management situation and without any hesitation we activated N-SIDE's risk management method. The process consists of 6 consecutive steps:
- Identify the risk
- Identify the causes
- Find risk mitigation ideas
- Prioritize them
- Simulate & asses their impact
- Implement all the relevant solutions
Once the process was activated, we were fast to act and managed to avoid the drug shortage situation without losing a single day. By using our simulation and optimization solutions and collaborating closely with our client, we could jointly choose the best way forward.
Read the case study now if you want to see how data-driven decisions led to avoid any risk altogether while ensuring neither patients nor timeline were impacted.





Other content that could be of interest

Case study
N-SIDE collaborates with AstraZeneca
N-SIDE and AstraZeneca worked together to reduce the waste and cost of this trial while still keeping the patients and their safety at the center of their concerns.
Read the case study to learn what they discovered and see the results they managed to reach. Overall, not only did they manage to reduce both the cost and drug waste levels of this study but some changes helped decrease the risk of missed dispensing for the patients as well.
download now
Webinar
Sustainability in Clinical Supplies: why is it an emergency ?
In this webinar, replay, discover through case studies how to navigate KPIs and trends that will strongly impact the future of clinical supply chain. You will see how sustainability, ethics, waste reduction, and faster timelines enable each other.
Watch now to discover what changes can be conducted in biopharma organisations to anticipate these changes
watch now

Solutions
Innovation that improves clinical supply planning for complex trials
Clinical trial forecasting and planning activities are extremely challenging. This is partly because the rising complexity and frequent changes in protocols lead to a high level of uncertainty in the trials.
Clinical trial supply solutions can simulate the complexities and uncertainties of protocols. They generate accurate demand forecasts and robust supply chain strategies, minimizing waste while ensuring no stockouts
read more



