N-SIDE & AstraZeneca collaborate to reduce trial's cost and drug waste


Earlier this year, N-SIDE's Alexis Davin and Antoine Remiot teamed up with AstraZeneca's Britta Claesson to present an interesting case study during the annual Arena International Clinical Trial Supply Nordics event.
Optimization during trial design helps reduce drug waste
In this case study, the team talks about:
- The most impactful drivers of waste
- AstraZeneca's journey
- The Trial Design Thinking Process applied to an oncology trial
Throughout their collaboration, N-SIDE and AstraZeneca worked together to reduce the waste and cost of this trial while still keeping the patients and their safety at the center of their concerns. This led them to suggest relevant scenarios challenging aspects of the trial design that were then discussed with all departments involved. The various scenarios brought up by N-SIDE's team and AstraZeneca's Study Design Specialist touched on three important characteristics of the trial design:
- The prediction of the randomization visit
- The way to cover potential titrations
- The kit design
Read the case study to learn what they discovered and see the results they managed to reach. Overall, not only did they manage to reduce both the cost and drug waste levels of this study but some changes helped decrease the risk of missed dispensing for the patients as well.





You might also like ...

solutions
Innovation that improves clinical supply planning for complex trials
Clinical trial forecasting and planning activities are extremely challenging. This is partly because the rising complexity and frequent changes in protocols lead to a high level of uncertainty in the trials.
Clinical trial supply solutions can simulate the complexities and uncertainties of protocols. They generate accurate demand forecasts and robust supply chain strategies, minimizing waste while ensuring no stockouts.
Read more
webinar
Oncology trials: supply chain planning doesn't have to be that complex!
In this webinar on demand, you will learn from the very people who are responsible for optimally satisfying oncology patient demand every day.
Get a better understanding of how clinical supply optimization algorithms, such as the N-SIDE Supply App, streamline and support the proactive management of oncology clinical supply.
get replay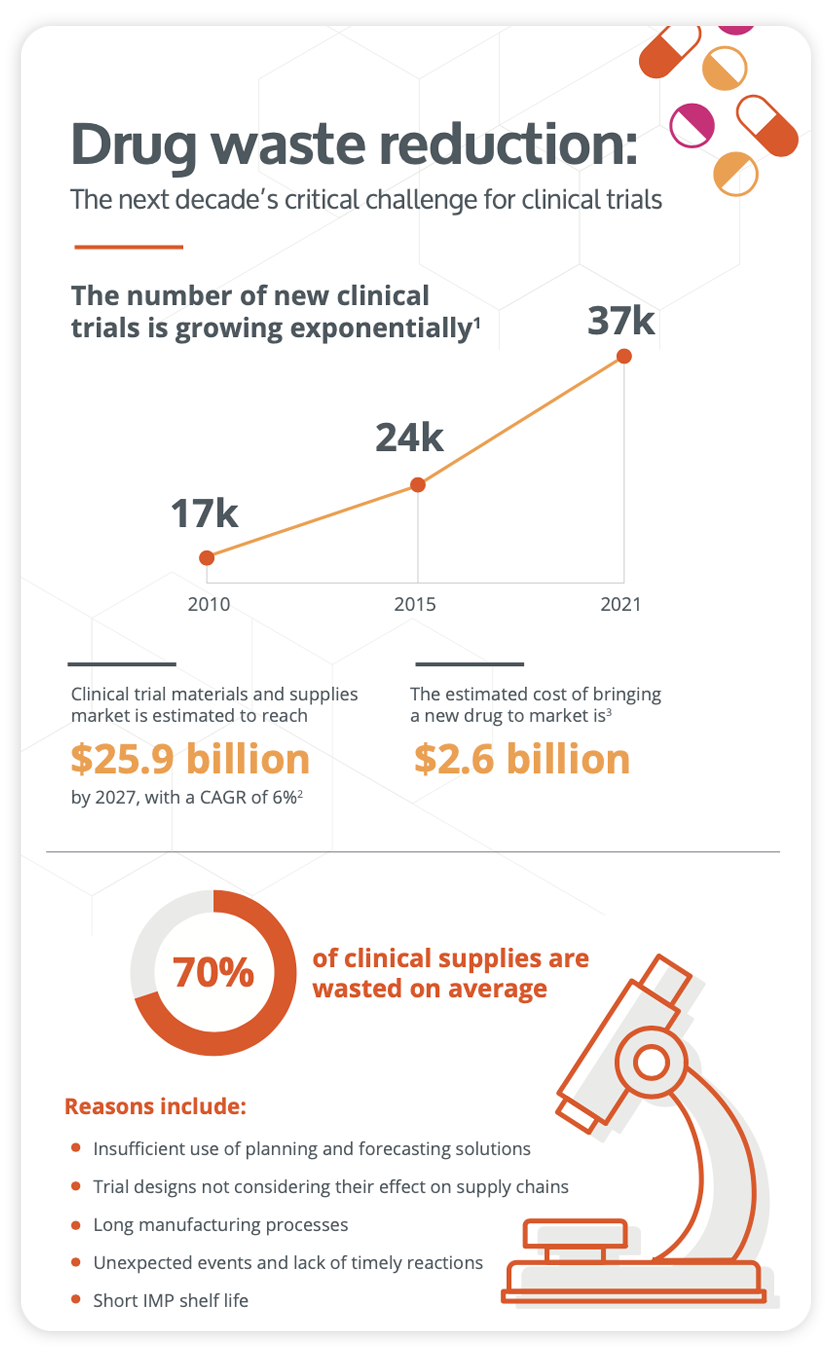
infographic
Curb drug waste and improve your clinical trial strategy
They are many factors that contribute to bottlenecks in the supply chain, including the increasing complexity of clinical trial designs, the shorter products shelf lives in innovative treatments, a severe shortage of manufacturing resources, etc.
See what clinical supply chain managers can do to curb these risks.
download now


