Maximize the supply friendliness of your clinical trial protocol – start optimizing at protocol design!

 Brieuc Triffaux
Brieuc Triffaux
Critical decisions are taken during protocol design. It is essential to maintain patient centricity and respect site constraints, but it is also an opportunity to evaluate the supply impact.
Different scenarios can be investigated with an advanced simulation and optimization tool that accurately models both the clinical design and the supply aspects of the protocol. This allows for assessing the supply impact of these early strategic decisions with quantified results to help inform decision making and increase team collaboration.
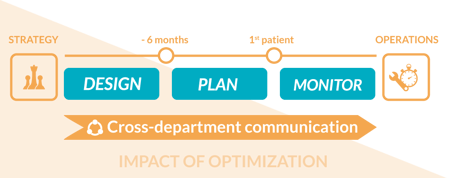
 Convention may say that the time to start work on optimizing study supply is about six months before the first patient is planned to enroll, allowing more information to be considered in the forecast assumptions before needing to order the packaging production quantities and setting up the IRT/distribution strategy. However, as consultants working on optimizing clinical supply strategies, we have seen the biggest impact of optimization by starting even earlier at the protocol design stage.
Convention may say that the time to start work on optimizing study supply is about six months before the first patient is planned to enroll, allowing more information to be considered in the forecast assumptions before needing to order the packaging production quantities and setting up the IRT/distribution strategy. However, as consultants working on optimizing clinical supply strategies, we have seen the biggest impact of optimization by starting even earlier at the protocol design stage.
Inform protocol design strategic decisions with simulation results
One may question why one would start with optimization during protocol design when so many aspects of the trial design could still change. But this is precisely the opportunity – to investigate different possibilities being considered and evaluate their impact with quantified results. An advanced simulation and optimization tool, such as N-SIDE’s solution Supply App (formerly CT-FAST), allows the study protocol to be accurately represented from both a clinical design and supply design perspective. It makes it then possible to assess different aspects of the protocol through simulation and compare the results of different scenarios.
Achieve a supply friendly protocol design
 Strategic decisions are taken during protocol design which have a direct impact on the drug supply over the entire trial. Consequently, decisions taken at this stage can impact the amount of drug product that is required to keep as buffers at depots and at sites in order to ensure that there is no risk of missing any patient dispensing during the trial. The amount of buffers needed impacts the amount of drug product that will eventually go to waste over the course of the trial. As trials are supplied with more and more expensive biotech drugs and comparators, and face limited internal resources for the investigational medicine needed across multiple studies, it is even more important to consider the supply aspects early on and investigate the best fit design for the trial. Investigating options at this time will allow for minimizing study overage and developing a supply friendly protocol – while still fully considering the other key aspects of keeping a patient and site friendly protocol.
Strategic decisions are taken during protocol design which have a direct impact on the drug supply over the entire trial. Consequently, decisions taken at this stage can impact the amount of drug product that is required to keep as buffers at depots and at sites in order to ensure that there is no risk of missing any patient dispensing during the trial. The amount of buffers needed impacts the amount of drug product that will eventually go to waste over the course of the trial. As trials are supplied with more and more expensive biotech drugs and comparators, and face limited internal resources for the investigational medicine needed across multiple studies, it is even more important to consider the supply aspects early on and investigate the best fit design for the trial. Investigating options at this time will allow for minimizing study overage and developing a supply friendly protocol – while still fully considering the other key aspects of keeping a patient and site friendly protocol.
Optimize your decisions during protocol design
There is a broad range of questions that can be investigated using simulation and optimization at the protocol design phase such as the:
- country list
- intervals between different events in the trial
- packaging design
- distribution network design
- home delivery of medication
Bringing expertise gained from working on numerous and different types of trials, N-SIDE consultants can help further identify the points to question during protocol design from a supply impact perspective. We repeatedly receive the feedback from our clients that having the quantified results helps teams to better collaborate. This allows to achieve a protocol that is designed to ensure patient centricity and respect sites constraints, while also optimizing supply aspects.
Case Study
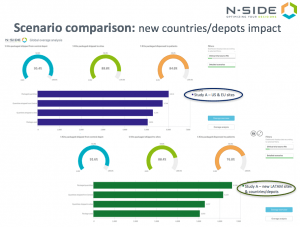
Clinical operations is considering to include countries in another region (LATAM) requiring local depots while keeping the same number of sites. Supply team is concerned regarding the feasibility to supply these extra countries with the constraints on the drug product without postponing the trial start date.
> Adding a local depot to the supply network increases the study overage. Extra drug product needs to be maintained at each local depot in case of urgent need, such as a loss of a site shipment due to a temperature excursion or when sites enroll much faster than expected.
> More risky situations – it is always tough to guarantee that your depot is sufficiently stocked when you have a long shipping lead time. It is hard to react to protect patient service levels.
N-SIDE simulations calculate the optimal amount of kits needed to supply the study for each scenario. The consequence on drug waste at each level of the supply chain is quantified and the opportunities to improve supply chain management are highlighted. You have all of the information in hand to engage your clinical colleagues and together take the best decisions for your trials and your patients!
Request a demo of our clinical trial optimization solution, N-SIDE Supply App, and learn about the benefits of optimization to facilitate clinical trial decisions!

