Going beyond forecasting with risk-based optimization


Read our publication “Clinical trial supply planning: Go Beyond Forecasting with Risk-based optimization” in the latest edition of Arena International’s Clinical Trial Supply Handbook!
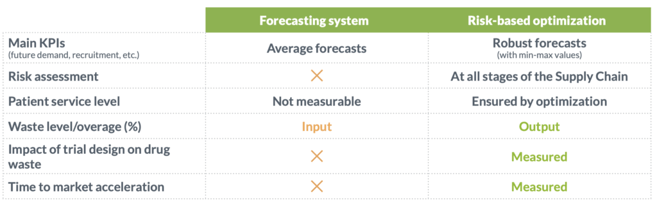
Discover the importance of properly distinguishing between traditional forecasting systems and risk-based optimization solutions in clinical trial supply management. These ‘hidden’ differences can have a direct impact on the ability of your clinical supply plan to ensure 100% patient services levels while also keeping drug waste to a minimum.
The main differences can be summarized as follows:
N-SIDE’s solution and its impact in clinical trial supply management
Reading our publication, you will understand why N-SIDE’s solution is capable of comprehensive risk assessment. Assessing risks at all stages of the clinical trial supply chain, from the earliest stages of manufacturing to patient dispensing is crucial to take a proactive role in risk mitigation. Additionally, you will learn how risk assessment is complemented by the fact that in our model, the overage level needed to safely supply your trial is an output from risk-based optimization. Thanks to risk-based optimization, your clinical trial supply management strategy can be optimized even when the protocol is still a draft, and continue throughout ongoing trial management.
Do not miss the opportunity to take a look at this piece with insights shared by our Life Sciences Solutions Adoption Team!
Interested in learning more about our solutions? Click below to learn more!



About the Author
Doménica Torres


